Objectives
Part I
1. To learn the technique of TLC and the visualization of colorless components.
2. To identify an unknown drug by a TLC comparison with standard compounds.
Part II
1. To learn the technique of column chromatography.
2. To separate the mixture of pyrene and p-nitroaniline by column chromatography.
Introduction
Chromatography is a method for separating complex samples into their constituent parts, and it is the most important procedure for isolating and purifying chemicals. There are few types of chromatography used to separate different complex samples. Types of chromatography commonly used included gas chromatography (GC), high performance liquid chromatography (HPLC), thin layer chromatography (TLC), and column chromatography (CC). In this experiment, we are only using the TLC and CC method to separate our sample mixtures. In chromatography, there are two phases involved in separating the samples, which are the stationary phase and mobile phase. Stationary phase is the part of the chromatographic system though which the mobile phase flows where distribution of the solutes between the phases occur. The stationary phase may be a solid or liquid that is immobilized or adsorbed on a solid. Examples of substances used as stationary phase included silica gel or alumina used in the TLC and CC, and filter paper was used as stationary phase in the paper chromatography. Alumina is generally used for chromatography of less polar compound whereas silica gel is better for compounds containing polar functional group. Mobile phase is the part of the chromatographic system which carries the solutes through the stationary phase. The mobile phases are either liquid or gases. The mobile phase is often known as eluent.
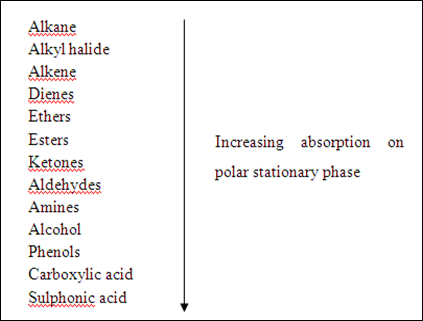
Solvent that can be used as eluent in TLC and CC can be categorized into three category: non-polar, moderate, and polar solvent. The following diagram shows some examples of solvent with different polarity

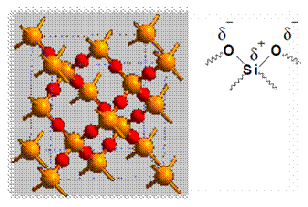
According to the principle of chromatography, different compounds will have different solubility and adsorption to the two phases between which they are to be partitioned. The samples are put on the stationary phase and are then carried along by the mobile phase. Since different compounds have different degrees of adsorption, the migration rate for each compound will be different and thus allow separation of compounds. The adsorption of compounds onto the stationary phase is depends on different types of interactions between the samples and the stationary phases. Examples of types of interactions included ion-dipole, dipole-dipole, hydrogen bonding, dipole-induced dipole, and Van Der Waals forces. In this experiment, silica gel was used as the stationary phase. The following diagram shows the structure of silica gel used as the stationary phase.
Silica gel is a porous form of SiO2 and the surface of gel contains Si-OH and Si-O-Si functional groups. As shown in the diagram above, the silica gel consists of polar functional group. Hence, the dominant interactive forces between the adsorbent (silica gel) and the materials to be separated are of dipole-dipole type. Highly polar molecules will interact fairly strong with the polar Si-O bonds in the silica gel and will tend to adsorb onto the fine particles of the adsorbent. The stronger adsorption will caused the material passes through the system to be slower. On the other hand, weakly polar molecules will held less tightly with the silica gel and thus tend to move through the adsorbent more rapidly than the polar species. The adsorption strengths of each compound having the following types of functional groups are arranged in the order of increasing group polarities:
However, variation may occur depending on the overall structure of each specific compound. The adsorption of polar molecules can be altered by using polar solvent. The more polar the eluent, the greater the eluting power, thus allowing compounds to move faster over the adsorbent surface. This is because polar solvent will compete for the silica adsorption sites, thus caused the polar molecules not to adsorb strongly on the silica gel. This caused all the compounds to travel at a faster rate but the order in which the compounds move remains the same. However, this will caused the separation between the non-polar compound and polar compound to become smaller and poor results might be obtained. Thus, a mixed eluent might be better for separating a mixture of samples consists of non-polar and polar compounds but the polarity of the mixed eluent must be controlled. Mixed eluent can be prepared by mixing low polarity and high polarity solvents and hence creating any eluting power needed.
In the TLC technique that we are going to applied in this experiment, a mixture of solvent ethyl acetate : hexane, 1 : 3 was used as the eluent. The silica gel (TLC plate) was used as the stationary phase. A capillary spotter was used to spot the samples on the TLC plate about 1.5 cm from the bottom of plate. The plate was then placed into a closed developing chamber which has a shallow layer of solvent that does not submerge the spot. The chamber is lined with a folded piece of filter paper to ensure a uniform and saturated atmosphere of solvent vapor. After the solvent has reached about 0.5 cm from the top of the plate, the plate was removed from the developing chamber. The capillary action of the solvent causes the initial spot to be separated into individual components that may be visualized by color identification or with the following techniques for colorless compound: (a) irradiation with ultraviolet light, (b) reversible staining with iodine vapor, and (c) spraying a reagent that irreversibly colors the spots. The rate at which a compound moves in respect to the solvent front, Rf, is characteristic of that compound under standard conditions. The Rf values can be calculate by using the following equation:
Rf = Distance of Compound / Distance of Solvent
TLC can be used to separate a very small amount of sample easily and rapidly without any costly equipment. Thus, it is often used to monitor the progress of a reaction by running the crude sample beside the reaction sample on the same plate. It also can be used to determine the best eluent for subsequent separation by column chromatography. Some of the common uses of TLC included:
(a) To determine the number of components in a mixture.
(b) To determine the identity of two substances.
(c) To monitor the progress of a reaction.
(d) To determine the effectiveness of a purification.
(e) To determine the appropriate conditions for a column chromatographic separation.
(f) To monitor column chromatography.
Column chromatography is a technique that uses an adsorbent packed in a glass column, and a solvent that moves down slowly through the packed column. Similar to TLC, silica gel was used as the stationary phase. The eluent used also the same which is the mixture of ethyl acetate : hexane in a ratio of 1 : 3. The column is packed with silica gel and the solvent was allowed to drain as the silica packs tightly. Small layer of sand was applied on top of the silica gel to avoid disturbance of silica gel during the adding of fresh solvent. The mixture of compounds was dissolved in small amount of solvent and poured into the column. The more polar compound will adsorb strongly on the silica gel and only move a little with every addition of fresh solvent. The less polar compound will move faster down the column due to less interaction with the silica gel. Fresh solvent is continually added to the top of the column until each band resolves and is carefully collected. The movement of solvent down the column due to the gravitational forces will caused the mixture of compounds to be separated. When the substances are colored, we can directly observe and collect them as they drain off. However, when the compound is colorless, several small fractions of the eluting solvent must be collected and testing each by thin layer chromatography.
Apparatus & Materials
Part I
UV lamp, capillary tube, 250 mL beaker, aspirin, acetaminophen, caffeine, unknown A, unknown B, TLC plates, ethyl acetate, hexane, and iodine.
Part II
Glass column, UV lamp, capillary tube, 250 mL beaker, test tubes, glass funnel, pyrene, p-nitroaniline, TLC plates, ethyl acetate, hexane, and iodine.
Procedures
Part I Analysis of Analgesic Drugs
Part A Spotting of the TLC plates
1. A TLC plate was obtained from the instructor.
2. The plate was set down on a clean, dry surface, and then a line was drawn lightly across the plate about 1.5 cm from the bottom and 0.5 cm from the top of the plate with the aid of a 2B pencil.
3. Five 2-3 mm lines was made, spaced about 0.6 cm apart and running perpendicularly through the line across the bottom of the TLC plate.
4. The first and the last line were drawn such that they are about 0.8 cm from the edge of the plate.
5. Separate capillary tube was used for spotting each sample on the TLC plate.
6. Acetaminophen solution was first spotted, then the caffeine, then the unknown A, then aspirin, and lastly the unknown B.
7. The spots are made as small as possible to avoid “tailing” when the plate was developed.
8. The plate was examined under the ultraviolet (UV) light to see that enough of each compound has been applied; if not, more sample were added.
Part B Developing the TLC plates
1. A developing chamber was developed using a 250 mL beaker as the chamber, a half-piece of filter paper inside, and aluminium foil to cover.
2. The eluent, 1 : 3 mixture of ethyl acetate : hexane, was poured into the beaker to a depth of under 1 cm (about 15 mL).
3. The prepared TLC plate was placed in the developing chamber and the solvent level was ensured to be below the pencil line.
4. After the solvent has risen to near the top of the plate (about 0.5 cm from the top), the plate was removed.
5. The solvent was allowed to evaporate from the plate in the fumehood.
Part C Visualization
1. The colorless compounds are visualized by illumination of the plate with an ultraviolet (UV) lamp.
2. The spots were outlined with a 2B pencil.
3. The spots may also be visualized by putting the plate in an iodine chamber for a couple minutes.
Part D Comparison of the unknown with reference standards
1. The plate was sketched in the notebook and the Rf values for each spot was calculated.
2. The unknown drug was determined based on the Rf value.
Part II The Separation of Pyrene and p-Nitroaniline by Column Chromatography
Part A Column preparation
1. A 40 cm chromatography column, 15 g of deactivated silica gel and 110 mL of the developing solvent mixture (ethyl acetate : hexane, 1 : 3) were obtained.
2. Slurry of the adsorbent (silica gel) was prepared with 50 mL of solvent in a 250 mL Erlenmeyer flask.
3. The column was clamped in a vertical position.
4. The stopcock of the column was closed and 15 mL of solvent was poured in.
5. After setting, all of the slurry was quickly decant through a funnel into the column.
6. The stopcock was opened and solvent was allowed to drain while the wall of the column was tapped with the ends of a folded piece of rubber tubing.
7. Once the solvent level is within 6 cm of the top of the adsorbent, a 0.5 cm level layer of sand was added on the adsorbent.
8. Excess solvent was drained off until its level is precisely on top of the sand and the stopcock was closed.
Part B Separation and collection of pyrene and p-nitroaniline
1. The pyrene (0.02 g) / p-nitroaniline (0.02 g) mixture was added with a few drops of ethyl acetate to dissolve as much of the mixture as possible.
2. The solution was carefully transferred to the top of the sand layer with a dropper.
3. The solvent was drained off until the mixture solution is just below the top of the sand.
4. The wall was rinsed with about 1 mL o fresh solvent (ethyl acetate : hexane, 1 : 3) and drained until the level is once again below the top of the sand.
5. The rinsing of the wall was repeated until the solvent above the silica gel is virtually colorless.
6. The column was filled with fresh solvent very carefully and the solvent was allowed to drain.
7. The separation of bands was observed as the column developed.
8. The colorless band of pyrene was collected into four fractions.
9. When the edge of the yellow band (p-nitroaniline) reached the lower part of column, a new test tube was replaced and the yellow band was collected into four fractions.
10. Each fraction was concentrated to a small volume by evaporation for analysis by TLC.
Part C Analysis of the fractions
1. The fractions were spotted on a TLC silica gel plate along with the reference pyrene and p-nitroaniline.
2. The TLC plate was developed in a developing chamber containing a 1 : 3 micture of ethyl acetate : hexane.
3. The TLC plate was visualized with the ultraviolet (UV) light to determine which fractions are pure pyrene and which are pure p-nitroaniline.
4. The chromatogram was drawn in the lab notebook.
5. The Rf values for pyrene and p-nitroaniline were calculated.
Results & Calculations
Part I Analysis of Analgesic Drugs
Distance of solvent travelled = 8.0 cm
Distance travelled by compounds: (a) Acetaminophen = 0.50 cm
(b) Caffeine = 0.35 cm
(c) Unknown A = 0.50 cm
(d) Aspirin = 2.40 cm
(e) Unknown B = 2.20 cm
Rf values of compounds: (a) Acetaminophen = 0.0625
(b) Caffeine = 0.0438
(c) Unknown A = 0.0625
(d) Aspirin = 0.3344
(e) Unknown B = 0.2750
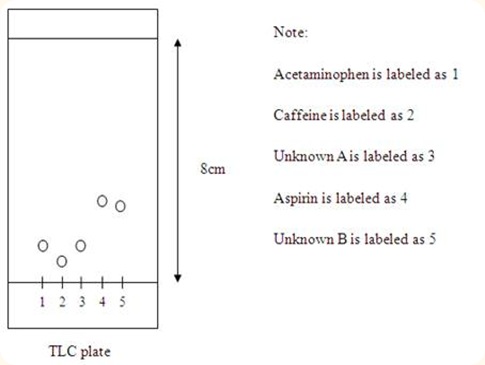
Diagram of acetaminophen, caffeine, and unknown A, aspirin, and unknown B travelled on the TLC plate
Conclusion: Unknown A belongs to the acetaminophen whereas unknown B belongs to the aspirin.
Part II The Separation of Pyrene and p-Nitroaniline by Column Chromatography
Distance of solvent travelled = 8.0 cm
Distance travelled by compounds: (a) Pyrene: (i) First fraction = 6.10 cm
(ii) Second fraction = 6.10 cm
(iii) Third fraction = 6.10 cm
(iv) Fourth fraction = 6.10 cm
(b) p-Nitroaniline: (i) First fraction = 1.60 cm
(ii) Second fraction = 1.70 cm
(iii) Third fraction = 1.70 cm
(iv) Fourth fraction = 1.70 cm
Rf values of compounds: (a) Pyrene: (i) First fraction = 0.7625
(ii) Second fraction = 0.7625
(iii) Third fraction = 0.7625
(iv) Fourth fraction = 0.7625
(b) p-Nitroaniline: (i) First fraction = 0.2000
(ii) Second fraction = 0.2125
(iii) Third fraction = 0.2125
(iv) Fourth fraction = 0.2125
Diagram of pyrene and p-nitoraniline travelled on the TLC plate
Conclusion: Pyrene is present in the 1st to 4th spots while p-nitroaniline is present in the 5th to 8th spots.
Discussion
The eluent used in this experiment is a mixture of ethyl acetate : hexane in a ratio of 1 : 3. Ethyl acetate, CH3COOCH2CH3, is a polar solvent because of the oxygen bonded to carbon in the compounds has higher electronegativity than carbon. So, the oxygen atom in the ethyl acetate will become partial negative while the carbon atom will acquire a partial positive charge. Hexane, CH3(CH2)4CH3, is an organic solvent which is non-polar. Mixture of these two solvents gives the eluent polarity properties and allows the polar compounds to be separated as well. If the polarity of the eluent is too low, the polar compounds will not be able to carry by the eluent and will not be separated. However, if the polarity of the eluent is too high, the polar compound will travel so fast that the separation between non-polar compound and polar compound to become so small, which result in poor separation. Hence, the polarity of the eluent must be optimal for the separation of the samples during chromatography. A few drops of acetic acid have been added to the eluent to prevent the deprotonation of the compounds during the separation of compounds. This is an important step for retain the identity of the compounds and hence, a pure compounds can be separated out.
In Part I of this experiment, TLC plate have been used for the separation of the compounds. Silica gels were coated on the TLC plate and act as the adsorbent. Silica gel consists of polar functional group such as Si-O-Si and Si-OH which results from the difference of electronegativity of the of oxygen atom with the silicon atom. Since the silica gel is polar, polar compounds will then interact strongly with the silica gel and adsorb on the silica gel strongly. This will caused the polar compounds to travel only a short distance from the bottom of the TLC plates as the eluent move upward. As for the non-polar compounds, they interact weakly with the silica gel and thus can be carried easily by the eluent. As the eluent moving upwards, the non-polar compounds can travel further away from the bottom of the TLC plate. The moving of eluent along the TLC plate is due to the capillary action of the solvent, allowing the solvent to travel in the gap present in the three dimensional network of silica gel. This causes the initial spot to be separated into individual components as the samples were carried by the eluent. A folded filter paper was inserted into the developing chamber to help the eluent move upwards quicker. This is because when eluent travels up along the filter paper, it will vaporize easily due to the larger surface area. Since the developing chamber is covered with aluminium foil, the eluent vapor will remain in the chamber. When the chamber is saturated with eluent vapor, the eluent will then move upwards quicker along the silica gel without vaporization. This promotes the rate of eluent travelled along the silica gel without affecting the separation of the compounds.
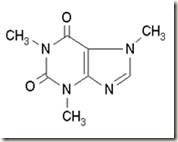
Three compounds which were spotted on the TLC plate are acetaminophen, caffeine and aspirin. The following diagrams show the structure of these compounds.
Acetaminophen Caffeine Aspirin
As the diagrams shown, all these three compounds are polar compounds. However, the polarities of the compounds may differ from each other. This is because of the electronegativity difference between the atoms in the compound. Nitrogen and oxygen atom present in these compounds have higher electronegativity compared to the carbon atom and hydrogen atom. Thus, nitrogen and oxygen atom will acquire a partial negative charge while the carbon and hydrogen atom will acquire a partial positive charge.
According to the results obtained, aspirin can travel the farthest away from the bottom of the TLC plate, followed by acetaminophen and caffeine. This is shown by the Rf values of each compound in which the higher Rf value indicate that the compound can travel further away and thus is less polar. The Rf value for aspirin is 0.3344, followed by acetaminophen, 0.0625 and finally the caffeine, 0.0438. Therefore, aspirin is the least polar compounds among those three compounds, followed by acetaminophen and finally the most polar compound is the caffeine. This may be due to the presence of large number of electronegativity atom such as nitrogen and oxygen atom in the compounds, thus increase the polarity of the caffeine compound. Since the compounds with similar polarities will travel with the same distance along the TLC plate, we can identify the unknown compounds by comparing the Rf values of the unknown with the standard compounds. Unknown A and B have the Rf values of 0.0625 and 0.2750 respectively. Unknown A has the same Rf value with the acetaminophen, thus we can conclude that unknown A is acetaminophen. Unknown B has almost similar Rf value with aspirin, so we can deduce that unknown B belongs to the aspirin.
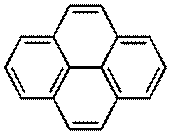
In Part II of this experiment, slurry of silica gel was used in column chromatography. The function of silica gel in the column is similar to the silica gel on the TLC plate, which act as the stationary phase for the materials to be separated to adsorb. The two compounds which were mixed together and separated by using column chromatography technique are pyrene and p-nitroaniline. The structures of the compounds are shown in the following diagram.
Pyrene p-Nitroaniline
According to the structure of pyrene, it is a non-polar compound. This is because only carbon and hydrogen atom present in the compound and the difference in electronegativity of carbon and hydrogen atom is very small only. On the other hand, p-nitroaniline consists of highly electronegativity atoms in its compound which is nitrogen and oxygen atom. This makes the p-nitroaniline a highly polar compound and thus will adsorb strongly to the silica gel. It was then can be predicted that pyrene will be collected first from the column before p-nitroaniline. This is because pyrene which is non-polar compound is weakly adsorb onto the silica gel and will be carried by the eluent easily as the eluent drained off from the column due to gravitational forces. The mixture of pyrene and p-nitroaniline was dissolved in minimum volume of ethyl acetate before added to the column. If too much of the solvent used, it will results in a poor separation of the compounds. This is because when the compounds dissolved in high volume of solvent, certain portions of the pyrene will be on top of the column while the other will be at the bottom of the column. This happened similarly to the p-nitroaniline and caused the separation of the pyrene and p-nitroaniline not complete, which results in poor separation.
According to the results obtained, the first four fractions of eluent collected in test tubes consist of pyrene only and the last four fractions contain p-nitroaniline only. This is proven by doing the TLC on the fractions of pyrene and p-nitroaniline collected. The first four fractions of test tubes containing pyrene were spotted on the TLC plate with four separate spots. Similar procedures were done to spot the p-nitroaniline in the last four fractions of test tubes on the same TLC plate. It was shown that pyrene move farther on the TLC plate compared to the p-nitroaniline. This proved that p-nitroaniline is more polar compared to the pyrene. However, the first and the fourth spot which are the spots of pyrene have a very fade color under the ultraviolet light. This might be due to low concentration of pyrene at the beginning and at the end of collection of colorless band before the yellow band was collected. At the beginning, most of the pyrene still moving from the top of the column and haven’t reach the bottom of the column. So the concentration of the pyrene is lower. At the end of collection of colorless band, since most of the pyrene already drained off, so there is lower concentration of pyrene as well. Same thing happened to the fifth and the eighth spot which are the spots of p-nitroaniline. The yellow color of p-nitroaniline is fader for the fifth and eighth spot because of lower concentration of p-nitroaniline. It was also observed that the four spots of pyrene on the TLC plate have mixed together during the TLC was carried out. This may be because of the pyrene is non-polar and thus interact weakly with the silica gel. When eluent passed through the spots, they were carried easily by the eluent and thus dissolved together to form a large spot on the TLC plate. Another reason might be due to human error in which the diameter of the spots were too big and thus the pyrene can easily interact with each other and mixed together.
Precaution Steps
1. Do not look directly at the ultraviolet lamp.
2. Do not interrupt the beaker after TLC plate has been introduced into the developing chamber.
3. Do not bend the silica gel excessively since the silica adsorbent may flake off.
4. Do not stop the collection of fractions of eluting solvent too long as the compounds may settle down to the bottom of the column and mixed together.




Thin layer chromatography can be used to observe the progress of a reaction, identify compounds present in a given mixture, and determine the purity of a substance. TLC method is simplest, easy and quick results given to the chemist as how many compounds are purify from the mixture. TLC is used in organic chemistry, a method of chemistry to identifying compounds.
ReplyDeleteBosan Menang tidak dibayar ? judi sabung ayam
ReplyDeleteOur trained chemists work on custom projects designed specifically for the needs of each client. N-ethylpyridinium bromide
ReplyDeleteTips dan Trick Bermain Judi Online
ReplyDeleteAgen Toto Macau
Museumtoto IDNLive
Museumbola Slot IDN
Museumbola Slot Pulsa
Museumbola Joker123
AKSES SEGERA SITUS KAMI 1 ID BANYAK PERMAINAN
WA OFFICIAL : +6283157394921
youtube :: youtube :: youtube :: youtube :: video_admins
ReplyDeleteyoutube :: youtube :: youtube youtube mp4 :: youtube :: youtube :: youtube :: youtube youtube :: youtube :: youtube :: youtube :: youtube youtube to mp4 youtube :: youtube :: youtube :: youtube 바카라사이트 :: youtube :: youtube_admins.
Casino Las Vegas - MapYRO
ReplyDeleteCasino 밀양 출장마사지 Las Vegas 제주도 출장안마 is situated in Las Vegas 안성 출장마사지 and features a wide variety of slot machines and table games 제천 출장샵 to suit your taste. The 목포 출장마사지 casino's hotel is