Objectives
1. To understand the enthalpy chemistry.
2. To determine the calorimeter constant.
3. To determine the enthalpy of reaction of acid-base reactions.
4. To study the exothermic reactions.
Introduction
Enthalpy is a measure of total energy of a thermodynamic system. It includes the internal energy of the system and the product of its volume multiplied by the pressure exerted on it by its surrounding.
H = U + p*V
H = Enthalpy p = Pressure
U = Internal Energy V = Volume
The enthalpy is normally measure with S.I. unit of Joule, J, although other units still in use such as Calorie, C and calorie, c. However, we often measure the change in enthalpy, ΔH instead of measuring the enthalpy, H because the total enthalpy cannot be measured directly.
Enthalpy change is defined by the following equation:
ΔH = HFinal – HInitial
ΔH = Enthalpy change
HFinal = Final enthalpy of the system. In a chemical reaction, HFinal is the enthalpy of the products.
HInitial = Initial enthalpy of the system. In a chemical reaction, HInitial is the enthalpy of the reactants.
Enthalpy change is in positive values in endothermic reaction and negative values in exothermic reaction. Exothermic reactions involve energy released as heat into its surroundings, causing the temperature of the surroundings to rise while endothermic reactions involve energy acquired from its surroundings as heat, causing the surrounding temperature to drop. Change in enthalpy that occurs as a result of a chemical reaction is numerically equal to the heat of reaction under constant (atmospheric) pressure conditions (ΔH = q). The heat of reaction is conveniently measured adiabatically in a Dewar calorimeter by the rise or fall in temperature of the products produced by the reaction in solution. Dewar flask is used because it is designed to preserve heat and minimize heat loss to the surrounding. In addition, isolated system is also required in this experiment to obtain an accurate data. Since every Dewar flask has different calorimeter constant because of different substances used, so the calorimeter constant ( ) must first be determined, that is the quantity of heat required to increase the temperature of the calorimeter and its content by 1 °C.
The constant is measured by supplying the calorimeter and contents with a definite known quantity of heat. This can be done electrically or by adding a known amount of concentrated sulphuric acid.
Results & Calculation
Part 1 Calorimeter Constant
By using the values calculated, 0.384M in Graph 2, we can estimate the amount of heat liberated which is 2.775 kJ. Since the heat liberated by the dilution of sulphuric acid is then absorbed by the Dewar flask, so the value of the ΔH is in positive value.
Therefore, the calorimeter constant has a value of 0.338 kJ / °C.
Part 2 Enthalpy of Reaction
Part I
Amount of heat absorbed by the Dewar flask after adding nitric acid to the mixture (50 c of water + 50 c of 1M sodium hydroxide) is 2.535 kJ. This means the total enthalpy of reaction, ΔH has a value of -2.535 kJ because the heat is being released from the reaction. However, the enthalpy changes during the dilution of nitric acid also need to be considered to obtain more accurate result.
Part II
Therefore, the enthalpy of reaction of sodium hydroxide with nitric acid is -2.028 kJ.
Discussion
The calorimeter constant for the Dewar flask used in this experiment has a value of 0.338 kJ / °C. This means that for every 0.338 kJ of energy absorbed by the Dewar flask, the contents of the Dewar flask will increase by 1 °C. However, this value is not so accurate because of possible errors during the experiment. In order to obtain an accurate result, the Dewar flask must be an isolated system. This is to ensure that all the heat released to or absorbed from the surrounding remain inside the Dewar flask but not from outside of the flask. However, there are no ideal Dewar flask in this world that guarantee no heat will be loss from the Dewar flask. This is because heat can travel through vacuum by radiation and radiation cannot be controlled. So, the design of the Dewar flask can only prevent conduction and convection of heat but only able to minimize the radiation of heat.
Besides, the top of the Dewar flask is also let to open in this experiment to ease the measurement of temperature of the contents in the Dewar flask. This further caused the error in data because the system is not isolated system anymore but became an open system. Open system allowed heat loss to or acquired from the surroundings and caused the temperature difference, ∆T to drop. During the titration, methyl orange was introduced into the solution before start titration. Methyl orange is used as an indicator of the equivalence point of the titration because it has a sharper end point and the change of colour is easier to be observed. The methyl orange is red in colour when the pH value is below 3.1 and yellow when above 4.4. Any pH value in between this range will show a mixture of those two colours.
From the experiment, we know that both the reaction between sodium hydroxide with sulphuric acid and sodium hydroxide with nitric acid are exothermic reaction. This is shown in the temperature differences where the temperature of the mixture increased after both reactions. This means that heat is being released from the system to its surrounding and caused the surrounding temperature to increase. In part 2, we do not take the value of Δ as the enthalpy of reaction between sodium hydroxide and nitric acid because dilution of nitric acid also can cause change in enthalpy. Instead, we take into consideration of enthalpy change of dilution of nitric acid to obtain a more accurate result.
Besides, some of the precaution steps have to be carried out such as wearing gloves and goggle during the experiment. The bottle of the concentrated nitric acid and sulphuric acid has to be closed when not in use and put in the fume hood because the white fumes of the nitric acid or sulphuric acid can be toxic.
Conclusion
Enthalpy change, ΔH can be used to measure the heat of reaction because they are numerically equal. (ΔH = q) Dewar flask is used to create an isolated system to ensure that the data more accurate. Since every Dewar flask has their calorimeter constant, so we need to measure that constant before the experiment start. The calorimeter constant of the Dewar flask used in this experiment has a value of 0.338 kJ / °C.
In part 2 of the experiment, enthalpy of reaction between sodium hydroxide and nitric acid were to be determined. The enthalpy of dilution of nitric acid need to be considered as it can affect the results. Enthalpy of dilution of nitric acid is -0.507 kJ. After deduct this enthalpy of dilution from the total enthalpy change during the reaction, we can get the enthalpy of reaction of sodium hydroxide with nitric acid which is -2.028 kJ. By measuring the enthalpy of reaction, the reaction between sodium hydroxide and nitric acid is to be known as exothermic reaction since the energy is being released to the surrounding. Dilution of nitric acid and sulphuric acid is also known as exothermic reaction as well.
References
1. Darrell D. Ebbing & Steven D. Gammon (2009). General Chemistry Ninth Edition. Boston, MA & New York, NY: Houghton Mifflin Company. Chapter 18, page 732.
2. Raymond Chang (2005). Physical Chemistry for the Bioscience. Edwards Brothers, Inc. Chapter 3, page 46 – 47.
3. Hans Kuhn, Horst-Dieter Forsteling & David H. Waldeck (2009). Principles of Physical Chemistry. New Jersey: John Wiley & Sons, Inc. Chapter 17, page 559.
Appendix
Part 1 Calorimeter Constant
Table 1 Temperature of 100 c of distilled water in Dewar flask
| Time, minutes | 1 | 2 | 3 | 4 | 5 |
| Temperature, °C | 22.8 | 22.8 | 22.8 | 22.8 | 22.8 |
Table 2 Temperature of solution after adding 2 c of concentrated sulphuric acid
| Time, seconds | 15 | 30 | 45 | 60 | 75 | 90 | 105 | 120 | 135 | 150 |
| Temperature, °C | 31.0 | 31.0 | 31.0 | 31.0 | 28.5 | 28.0 | 28.0 | 28.0 | 28.0 | 28.0 |
| Time, seconds | 165 | 180 | 195 | 210 | 225 | 240 | 255 | 270 | 285 | 300 |
| Temperature, °C | 28.0 | 28.0 | 28.0 | 28.0 | 28.0 | 28.0 | 28.0 | 28.0 | 28.0 | 28.0 |
Table 3 Heat liberated when various quantities of concentrated sulphuric acid are added to 100 c of water
| Acid added, c | Molarity of solution, M | Heat liberated, kJ |
| 0.60 | 0.108 | 0.802 |
| 0.75 | 0.138 | 1.016 |
| 1.50 | 0.276 | 1.987 |
| 2.30 | 0.421 | 3.016 |
| 2.50 | 0.459 | 3.293 |
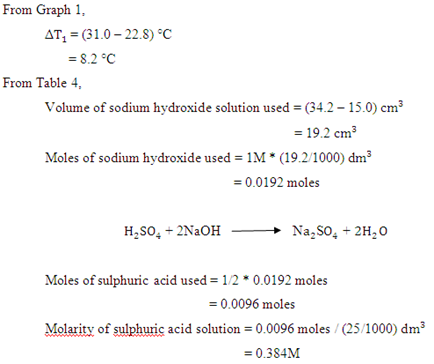
Table 4 Titration of 25 c of sulphuric acid solution against 1M sodium hydroxide.
| Initial burette reading, c | 15.0 |
| Final burette reading, c | 34.2 |
Part 2 Enthalpy of Reaction
Part I
Table 5 Temperature of mixture of 50 c of water and 50 c of 1M sodium hydroxide
| Time, minutes | 1 | 2 | 3 | 4 | 5 |
| Temperature, °C | 22.0 | 22.0 | 22.0 | 22.0 | 22.0 |
Table 6 Temperature of the mixture after adding 5 c of 10M nitric acid
| Time, seconds | 15 | 30 | 45 | 60 | 75 | 90 | 105 | 120 | 135 | 150 |
| Temperature, °C | 29.5 | 29.5 | 29.5 | 29.5 | 29.5 | 29.5 | 29.5 | 29.5 | 29.5 | 29.5 |
| Time, seconds | 165 | 180 | 195 | 210 | 225 | 240 | 255 | 270 | 285 | 300 |
| Temperature, °C | 29.5 | 29.5 | 29.5 | 29.5 | 29.5 | 29.5 | 29.5 | 29.5 | 29.5 | 29.5 |
Part II
Table 7 Temperature of 100 c of distilled water
| Time, minutes | 1 | 2 | 3 | 4 | 5 |
| Temperature, °C | 21.5 | 21.5 | 21.5 | 21.5 | 21.5 |
Table 8 Temperature of the water after adding 5 c of 10M nitric acid
| Time, seconds | 15 | 30 | 45 | 60 | 75 | 90 | 105 | 120 | 135 | 150 |
| Temperature, °C | 23.0 | 23.0 | 23.0 | 23.0 | 23.0 | 23.0 | 23.0 | 23.0 | 23.0 | 23.0 |
| Time, seconds | 165 | 180 | 195 | 210 | 225 | 240 | 255 | 270 | 285 | 300 |
| Temperature, °C | 23.0 | 23.0 | 23.0 | 23.0 | 23.0 | 23.0 | 23.0 | 23.0 | 23.0 | 23.0 |




As a global Contract Research Organization (CRO), headquartered in New York, USA, Alfa Chemistry has served the pharmaceutical and biotechnology industries for eight years. 1-dodecyl-2,3-methylimidazolium bromide
ReplyDelete